Description
Detailed Description:
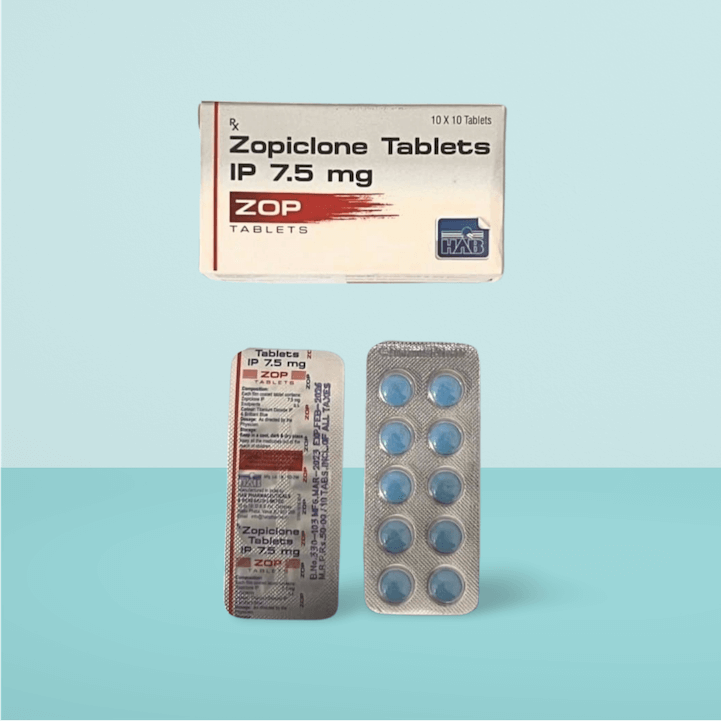
Zopiclone BLUE 7.5mg (Zopiclone Tablets IP 7.5 mg) is a non-benzodiazepine hypnotic medication designed to treat short-term insomnia and sleep disturbances. It works by increasing the activity of gamma-aminobutyric acid (GABA), a neurotransmitter that calms the nervous system, helping to induce and maintain sleep.
This medication is particularly beneficial for individuals struggling with difficulty falling asleep, frequent awakenings, or early morning waking. It enhances overall sleep quality, allowing users to wake up feeling refreshed and restored.
Key Benefits:
- Helps fall asleep faster and stay asleep longer
- Reduces nighttime awakenings and improves sleep duration
- Promotes relaxation by calming overactive brain activity
- Short-acting with minimal residual effects the next morning
- Non-benzodiazepine with a lower risk of dependence when used short-term
Dosage & Usage:
- Take as prescribed by a healthcare provider.
- Typically taken orally 30-60 minutes before bedtime.
- Swallow the tablet whole with water; do not crush or chew.
- Recommended for short-term use (7-14 days) to avoid dependence.
Precautions:
- Avoid alcohol and other CNS depressants while taking Zopiclone.
- May cause drowsiness, dizziness, or an unpleasant metallic taste.
- Not intended for long-term use due to potential dependency.
- Consult a doctor before use if pregnant, breastfeeding, or having a history of liver or respiratory conditions.
Storage:
Store in a cool, dry place away from direct sunlight and moisture. Keep out of reach of children.

Reviews
There are no reviews yet.